Theme: Discover the Difference and Develop the Possibilities for Shaping Future
PharmaTech 2017
Track 1: Pharmaceutics & Biopharmaceutics:
Biopharmaceutics examines the inter relationship of the physical/chemical properties of the drug, the dosage form (drug product) in which the drug is given, and the route of administration on the rate and extent of systemic drug absorption. It covers topics like Biopharmaceutical Technology, Therapeutic Biologics, Biologics Manufacturing, Pharmaceutical Packaging, Bioequivalence studies.
Pharmaceutics is a study concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. It covers topics like pharmacokinetics, toxicokinetics, pharmacodynamics, pharmacogenetics and pharmacogenomics, and pharmaceutical formulation. Also recent advances like pharmaceutical drug delivery systems, novel delivery forms, novel dosage forms, biotechnology and drug design.
Related Conferences:
8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany; 6th FIP Pharmaceutical Sciences World Congress 2017; Next Generation Pharmaceutical Samples; International Pharmacy Scholars Conference; 77th FIP World Congress of Pharmacy and Pharmaceutical Sciences; International Transporter Consortium (ITC) Workshop 3 - Transporters in Drug Development Pre-conference.
Track 2: Drug Targeting & Drug Development
Identifying drug targets plays essential roles in designing new drugs and combating diseases. Unfortunately, our current knowledge about drug targets is far from comprehensive. Screening drug targets in the lab is an expensive and time-consuming procedure. In the past decade, the accumulation of various types of study of science related data makes it possible to develop computational approaches to predict drug targets.
Topics covered include Targeted Therapy VS Immunotherapy, Targeted lung cancer therapy, Cancer treatment, Tumour Targeted Therapy
Related Conferences:
International Conference and Meeting on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany; Implementation of Biowaivers based on the Biopharmaceutics Classification System; Pharmaceutical Compliance Congress; The Pharma Commercial & Sales Conference; 6th Annual Pharma IPR Conference 2017; 3rd Anti-Counterfeiting Pharma Conference
Track 3: Pharmaceutical Research:
Pharmaceutical Research covers research spanning the entire spectrum of drug discovery, development, evaluation, and regulatory approval. Small drug molecules, biotechnology products including genes, peptides, proteins and vaccines, and genetically engineered cells are an integral part of recent published papers.
The recent advances in the field of Pharmaceuticals is majorly concentrated on smart drug delivery systems namely controlled drug delivery systems, gene delivery, protein delivery and vaccine delivery.
Related Conferences:
3rd International Conference on Advanced Clinical Research and Clinical Trials; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany ; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia;10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; International Transporter Consortium (ITC) Workshop 3 - Transporters in Drug Development Pre-conference; Implementation of Biowaivers based on the Biopharmaceutics Classification System; Pharmaceutical Compliance Congress; The Pharma Commercial & Sales Conference; 6th Annual Pharma IPR Conference 2017; 3rd Anti-Counterfeiting Pharma Conference.
Track 4: Pharmaceutical Materials:
Pharmaceutical materials are substances that are used in medical devices or in contact with biological systems. The Quality control of raw materials in pharmaceuticals has been a major setback in the pre formulation studies of API’s, hence many recent pharmaceutical research organization have taken a step to overcome these aspects by thorough quality control techniques. Biomaterials use impression from medicine, biology, chemistry, materials science and engineering. Although biomaterials are primarily used for medical applications, they are also used to multiply cells in culture, to assay for blood proteins in the clinical laboratory, in proceeding biomolecules in biotechnology, for fertility regulation implants in cattle, in diagnostic gene arrays, in the adequacy of oysters and for investigational cell-silicon "biochips." The commonality of these applications is the interaction between biological systems and simulated or modified natural materials.
Related Conferences:
5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia;9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 6th FIP Pharmaceutical Sciences World Congress 2017; Next Generation Pharmaceutical Samples; International Pharmacy Scholars Conference; 77th FIP World Congress of Pharmacy and Pharmaceutical Sciences; International Transporter Consortium (ITC) Workshop 3 - Transporters in Drug Development Pre-conference.
Track 5: Pharmaceutical Formulations:
Pharmaceutical Formulation is the process in which different chemical substances i.e., active chemical substances will combined together to produce a medical compound i.e., medical drug. This process involves production of drug which characterized by two things: Stability of the product, second Acceptability to the patient. Formulation studies focuses on factors like particle size, polymorphism, pH and solubility, in order to check whether these factors will effect on bioavailability of the drug or not. Pharmaceutical Formulations include Ophthalmic Formulation, Paediatric Formulation Development, Topical Formulation and Medication Formulation.
Largest Pharmaceutical Companies play a major role in API synthesis, Pharmaceutical Research and Development, Pharmaceutical Product Development, Types of drug formulations
Related Conferences:
9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany ; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; 6th FIP Pharmaceutical Sciences World Congress 2017; Next Generation Pharmaceutical Samples; International Pharmacy Scholars Conference; 77th FIP World Congress of Pharmacy and Pharmaceutical Sciences; International Transporter Consortium (ITC); Workshop - Transporters in Drug Development Pre-conference.
Track 6: Pharmaceutical Biotechnology:
Pharmaceutical Biotechnology is the science that covers all technologies required for producing, manufacturing and registration of biological drugs. Pharmaceutical Biotechnology is an increasingly important area of science and technology. The Pharmaceutical Biotechnology is widely spread, ranging from many ethical issues to changes in healthcare practices and a significant contribution to the development of national economy. Biotech drug makers essentially use those microorganisms or highly complex proteins from genetically-modified living cells as components in medications to treat various diseases and conditions, from cancer to rheumatoid arthritis to multiple sclerosis. Unlike pharma companies, biotechnology focus primarily on research and development, this begins with the discovery of novel compounds, which then convoy into the clinic for further testing.
Related Conferences:
International Conference on Biotech Pharmaceuticals December 07-09, 2017 Paris, France; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany; 6th FIP Pharmaceutical Sciences World Congress 2017; Next Generation Pharmaceutical Samples; International Pharmacy Scholars Conference; 77th FIP World Congress of Pharmacy and Pharmaceutical Sciences; International Transporter Consortium (ITC); Workshop - Transporters in Drug Development Pre-conference.
Track 7: Pharmaceutical Nanotechnology:
Nano pharmaceuticals offer the ability to detect diseases at much earlier stages and the diagnostic applications could build upon conventional procedures using nanoparticles. Nano pharmaceuticals represent an emerging field where the sizes of the drug particle or a therapeutic delivery system work at the Nano scale. In the pharmaceutical industry, a long standing issue is the difficulty of delivering the appropriate dose of a particular active agent to specific disease site. Nano pharmaceuticals have enormous potential in addressing this failure of traditional therapeutics which offers site-specific targeting of active agents. Such precision targeting via Nano pharmaceuticals reduces toxic systemic side effects, resulting in better patient compliance. In today world economy, a pharmaceutical industry faces enormous pressure to deliver high-quality products to patients while maintaining profitability. Therefore pharmaceutical companies are applying nanotechnology to enhance or supplement drug target discovery, Pharmaceutical product development and drug delivery.
Related Conferences:
17th International Conference and Exhibition on Nanomedicine and Nanotechnology in Health Care Nov 23-24, 2017 Melbourne, Australia; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany ; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; ; Implementation of Biowaivers based on the Biopharmaceutics Classification System; Pharmaceutical Compliance Congress; The Pharma Commercial & Sales Conference; 6th Annual Pharma IPR Conference 2017; 3rd Anti-Counterfeiting Pharma Conference; International Transporter Consortium (ITC) Workshop 3 - Transporters in Drug Development Pre-conference.
Track 8: Pharmaceutical Technologies:
Pharmaceutical technology is the appliance of scientific knowledge or technology to pharmacy, pharmacology, and the pharmaceutical industry. It includes design, techniques, and instrumentation in the manufacture, preparation, compounding, dispensing, packaging, and accumulating of narcotic and other preparations used in diagnostic and determinative procedures and in the treatment of patients.
Related Conferences:
International Conference on Pharmaceutical and Biomedical Engineering October 16-17, 2017 Osaka, Japan; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany ; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; Implementation of Biowaivers based on the Biopharmaceutics Classification System; Pharmaceutical Compliance Congress; The Pharma Commercial & Sales Conference; 6th Annual Pharma IPR Conference 2017; 3rd Anti-Counterfeiting Pharma Conference; International Transporter Consortium (ITC); Workshop - Transporters in Drug Development Pre-conference.
Track 9: Pharmaceutical Manufacturing:
Drug manufacturing (Pharmaceutical Manufacturing) is the process of industrial-scale synthesis of pharmaceutical drugs by pharmaceutical companies. The process of drug manufacturing can be broken down into a series of unit operations, such as milling, granulation, coating, tablet pressing, and others. Start-up Pharmaceutical Companies, drug manufactures, contract pharmaceutical companies, pharmaceutical marketing agencies all play a major role in drug synthesis from its raw material to the final finished product.
Parenteral drug delivery is the second largest segment of this transformative pharmaceutical market eclipsed only by the more mature oral solid dosage forms accounting for nearly 30 percent of total Pharma market share. According to Survey, the market for parenteral drug delivery products is projected to rise over 10 percent annually to $86.5 billion in 2019.
Related Conferences:
International Conference and Meeting on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany; International Transporter Consortium (ITC) Workshop 3 - Transporters in Drug Development Pre-conference; Implementation of Biowaivers based on the Biopharmaceutics Classification System; Pharmaceutical Compliance Congress; The Pharma Commercial & Sales Conference; 6th Annual Pharma IPR Conference 2017; 3rd Anti-Counterfeiting Pharma Conference.
Track 10: Pharmaceutical Engineering:
Pharmaceutical engineering is a branch of pharmaceutical science and technology that involves development and manufacturing of products, processes, and components in the pharmaceuticals industry (i.e. drugs & biologics). While developing pharmaceutical products involves many interrelated disciplines (e.g. medicinal chemists, analytical chemists, clinicians/pharmacologists, pharmacists, chemical engineers, biomedical engineers, etc.), the specific subfield of "pharmaceutical engineering" has only emerged recently as a distinct engineering discipline. This now brings the problem-solving principles and quantitative training of engineering to complement the other scientific fields already involved in drug development.
Related Conferences:
9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany ; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; Implementation of Biowaivers based on the Biopharmaceutics Classification System; Pharmaceutical Compliance Congress; The Pharma Commercial & Sales Conference; 6th Annual Pharma IPR Conference 2017; 3rd Anti-Counterfeiting Pharma Conference; International Transporter Consortium (ITC) Workshop 3 - Transporters in Drug Development Pre-conference.
Track 11: Drug Delivery Technologies:
With Drug Delivery Technology the unprecedented progresses of biomedical nanotechnology during the past few decades, conventional drug delivery systems (DDSs) have been involved into smart DDSs with stimuli-responsive characteristics. To enhance their therapeutic effects and reduce the related side effects, active drug molecules should selectively accumulate in the disease area for a prolonged period with high controllability. In comparison to the conventional DDSs, the smart controlled DDSs can effectively reduce the dosage frequency, while maintaining the drug concentration in targeted organs/tissues for a longer period of time. In this sense, the controlled DDSs provide broad insights and fascinating properties for decreasing drug concentration fluctuation, reducing drug toxicities and improving therapeutic efficacy.
Related Conferences:
11th World Drug Delivery Summit, October 16-18, 2017 (10 Plenary Forums - 1Event) Baltimore, Maryland, USA12th Annual Pharma Middle East Congress Sep 25-27, 2017 Dubai, UAE; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany ; 8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; Implementation of Biowaivers based on the Biopharmaceutics Classification System; Pharmaceutical Compliance Congress; The Pharma Commercial & Sales Conference; 6th Annual Pharma IPR Conference 2017; 3rd Anti-Counterfeiting Pharma Conference; International Transporter Consortium (ITC) Workshop 3 - Transporters in Drug Development Pre-conference.
Track 12: Medical Devices:
Instrument, apparatus, tool, software, material or other article, whether used alone or in combination used specifically for diagnostic or for therapeutic purposes and necessary for its proper application, intended for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease are medical devices.
Related Conferences:
8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany; 6th FIP Pharmaceutical Sciences World Congress 2017; Next Generation Pharmaceutical Samples; International Pharmacy Scholars Conference; 77th FIP World Congress of Pharmacy and Pharmaceutical Sciences; International Transporter Consortium (ITC) Workshop - Transporters in Drug Development Pre-conference
Track 13: Drug Regulatory Affairs:
Regulatory affairs (RA) also called government affairs, is a profession within regulated industries, such as pharmaceuticals, medical devices, energy, banking, telecom etc. Regulatory affairs also have a very specific meaning within the healthcare industries (pharmaceuticals, medical devices, biologics and functional foods). Regulatory affairs is a comparatively new profession which developed from the desire of governments to protect public health by controlling the safety and efficacy of products in areas including pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines. Regulatory Affairs is involved in the development of new medicinal products from early on, by integrating regulatory principles and by preparing and submitting the relevant regulatory dossiers to health authorities.
Regulatory Affairs is actively involved in every stage of development of a new medicine and in the post-marketing activities with authorized medicinal products. The Regulatory Affairs department is an important part of the organizational structure of pharmaceutical industry. Internally it liaises at the interphase of drug development, manufacturing, marketing and clinical research. Externally it is the key interface between the company and the regulatory authorities.
Related Conferences:
8th Global Pharmacovigilance & Drug Safety Summit, July 06-08, 2017 Kuala Lumpur, Malaysia; 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore; International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan; 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand; 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, July 24-26, 2017 Melbourne, Australia; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 17-18, 2017 Munich, Germany; 6th FIP Pharmaceutical Sciences World Congress 2017; Next Generation Pharmaceutical Samples; International Pharmacy Scholars Conference; 77th FIP World Congress of Pharmacy and Pharmaceutical Sciences; International Transporter Consortium (ITC) Workshop - Transporters in Drug Development Pre-conference.
Track 14: Pharmaceutical Management:
Pharmaceutical Management is learning the business and management side of health care and the pharmaceutical industry. It provides strong focus on the basic sciences, combining biological and pharmaceutical science coursework with marketing and general management studies. Topics include pharmaceutical sales; health care and health information management; food, drug and medical device industry regulatory oversight; and pharmacy distribution systems development and implementation.
Pharmaceutical management professionals make business and financial decisions regarding research and development of new medicine, marketing, and sales. They decide which types of medication should be developed and how they will be marketed to the public once they have been approved by the Food and Drug Administration (FDA).
Conference Series is a renowned organization that organizes highly notable pharmaceutical conferences and healthcare conferences throughout the globe. ConferenceSeries Ltd invites all the participants from all over the world to attend “International Conference and Exhibition on Pharmaceutical Development and Technology” during April 24-26, 2016 in Dubai, UAE.Which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
Pharma Tech 2017 is a latest technological platform to aid efficient drug discovery and development. These platforms range from vast chemical libraries, ultra-high throughput screening and huge genetic databases in discovery, to predictive toxicology platforms and even deep-seated knowledge of particular therapeutic areas in development. New research platforms designed to help its teams of investigators focus on a set of key strategic initiatives that will help guide their multibillion-dollar drug development program to advance new medicines that can get ahead of a disease and prevent it from taking a toll.
Pharmaceutical technology is a collective term for technologies to develop candidate compounds that have either been discovered or created into commercial pharmaceutical products. These products are made by transforming chemical compounds with useful effects on the human body into high-quality dosage forms that can appropriately exhibit effects against disease. Pharmaceutical technologies are divided into the following three functions.
Process technology for researching synthetic methods to be used to manufacture candidate compounds efficiently and consistently in large amounts and with high quality. Formulation technology for investigating dosage forms, formulations, and packages based on absorption stability, and usability in consideration of the characteristics of candidate compounds, and then selecting and preparing the optimal administration form. Analytical and quality evaluation technology for establishing a variety of analytical and quality evaluation systems to properly and appropriately assure the quality of the pharmaceutical products.
Efficacy and safety are the primary requirements for any pharmaceutical product. However, it is becoming significantly more important to provide pharmaceutical products that can be more easily used by patients, healthcare professionals, and caregivers in order to respond to the rapid aging of society and the needs for advanced medical care. Examples of user-friendly pharmaceutical products include extended-release tablets, which reduce the frequency at which pharmaceuticals must be administrated, and orally disintegrating (OD) tablets, which can be taken without water. Meanwhile, examples of innovation for healthcare professionals include syringes that are prefilled with drug solutions to reduce the hassle of preparation and the risk of needle injuries, as well as IC tags for pharmaceutical products or packages that are helpful in preventing medical errors. Additionally, we are utilizing various formulation technologies to provide user-friendly pharmaceutical products adding new value. Such innovations on this front include package designs and tablets with the product name printed on them in order to prevent any misuse.
We are advancing the development of new synthetic processes based on the eco-friendly concept of “green chemistry,” which is aimed at achieving global environmental sustainability through means such as preventing pollution and reducing consumption of materials and energy.
Why to attend???
With members from around the world focused on learning about innovations, latest technologies, different drug formulations for effective treatment and new drug moieties through pharmaceutical technology and its importance, this is your best opportunity to reach the largest assemblage of participants from the Clinical Research organizations, Pharmaceutical companies and technology related societies Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new research developments, and receive name recognition at this 3-day event. World-renowned speakers, the most recent issues, approaches, and the novel trends in Pharmaceutical Technology are hallmarks of this conference
Major sessions included at this conference are Pre-formulation studies, Drug Formulation Procedures, Drug Product Manufacturing, Pharmaceutical Engineering, Pharmaceutical Manufacturing, Pharmaceutical Technology, Technologies in Drug Delivery, Types of Pharmaceutical Formulations, Regulatory Requirements for Pharmaceuticals, Thermodynamics, Pharmaceutical Management.
.
Scope and Importance:
Pharmaceutical Technology is a latest technological platform to aid efficient drug discovery and development. These platforms range from vast chemical libraries, ultra-high throughput screening and huge genetic databases in discovery, to predictive toxicology platforms and even deep-seated knowledge of particular therapeutic areas in development. New research platforms designed to help its teams of investigators focus on a set of key strategic initiatives that will help guide their multibillion-dollar drug development program to advance new medicines that can get ahead of a disease and prevent it from taking a toll .Pharmaceutical research indulges in making medicines from plant- and chemical-based compounds. They work to prevent the spread of disease, ease pain, cure illnesses, and slow the effects of aging, among many other things. Pharmaceutical companies' mission to discover the next ground breaking medicine is a big cause steeped in big bucks, big competition, and, at times, big controversy. Insiders refer to the handful of multinational giants that dominate the industry-Pfizer and GlaxoSmithKline, to name two of the largest-as Big Pharma.
About Venue:
Dubai is the most populous city in the United Arab Emirates (UAE). It is located on the southeast coast of the Persian Gulf and is one of the seven emirates that make up the country. The city of Dubai is located on the emirate’s northern coastline and heads up the Dubai-Sharjah-Ajman metropolitan area.Dubai is to host World Expo 2020.
Dubai has emerged as a global city and business hub of the Middle East. It is also a major transport hub for passengers and cargo. By the 1960s, Dubai’s economy was based on revenues from trade and, to a smaller extent, oil exploration concessions, but oil was not discovered until 1966. Oil revenue first started to flow in 1969. Dubai’s oil revenue helped accelerate the early development of the city, but its reserves are limited and production levels are low: today, less than 5% of the emirate’s revenue comes from oil. The emirate’s Western-style model of business drives its economy with the main revenues now coming from tourism, aviation, real estate, and financial services. Dubai has recently attracted world attention through many innovative large construction projects and sports events. The city has become iconic for its skyscrapers and high-rise buildings, in particular the world’s tallest building, the Burj Khalifa.
Dubai provides a world class education and also enormous job opportunities. The most well-known universities in Dubai are American University in Dubai, Hult International Business School, Al Ghurair University, The American College of Dubai, University of Wollongong in Dubai, British University in Dubai offering courses in Business Administration, Engineering, Architecture and Interior Design.
American University in Dubai is one of the six UAE universities featured in QS World University Rankings 2014/2015. In 2013 Synergy University Dubai Campus opened its campus in Jumeirah Lakes Towers being a first University in Dubai to located outside of Educational Zones.
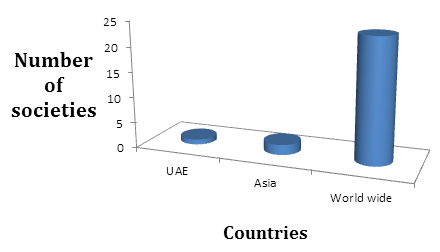
Societies in United Arab Emirates:
- Saudi Pharmaceutical Society or Kuwait Pharmaceutical Association
Societies and Associations in Asia:
- Federation of Asian Pharmaceutical Associations (FAPA)
- Asia Partnership Conference of Pharmaceutical Associations(APAC)
Societies World Wide
- Controlled Release Society (CRS)
- Institute of Pharmacy Management International (IPMI)
- International Academy of Compounding Pharmacists (IACP)
- International Association for Pharmaceutical Technology (APV)
- International eHealth Association
- International Federation of Pharmaceutical Manufacturers Associations (IFPMA)
- International Federation of Pharmaceutical Wholesalers (IFPW)
- International Pharmaceutical Excipients Council (IPEC)
- International Pharmaceutical Federation (FIP)
- International Pharmaceutical Students Federation (IPSF)
- International Society for Medical Publication Professionals (ISMPP)
- International Society of Oncology Pharmacy Practitioners (ISOPP)
- International Society for Pharmaceutical Engineering (ISPE)
- International Society for Pharmacoepidemiology (ISPE)
- International Society for the History of Pharmacy (ISHP)
- International Society for the Study of Xenobiotics (ISSX)
- International Society of Pharmaceutical Compounding (ISPhC)
- International Young Pharmacists' Group (YPG)
- Parenteral Drug Association (PDA)
- Regulatory Affairs Professionals Society (RAPS)
- Society for Biomolecular Sciences (SBA)
- Society for Cell Science (SFCS)
- Society for Translational Oncology (STO)
Statistical Representation:
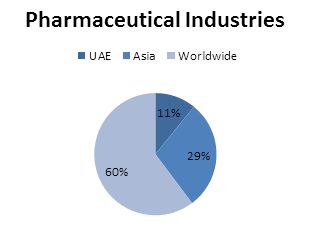
Pharmaceutical Industries in United Arab Emirates:
- Pfizer UAE
- Johnson & Johnson Middle East
- Merck UAE
- Novo Nordisk Pharma Gulf
- Gulf Pharmaceutical Industry-Julphar (JULPHAR)
- Smart Health and Nutrition Ltd.
- Hikma Pharmaceuticals PLC
- Valeant Pharmaceuticals MEA FZ LLC
- Al-Hayat Pharmaceuticals
- Amgen
- Elis Pharmaceuticals Limited
- Alliance Global
- Sanofi Aventis UAE
- Genpharm
- Ranbaxy Laboratories Limited
- BioPharma
- GlaxoSmithKline
- Gulf Inject
- Saga Pharmaceuticals
- Global Pharma
- Astra Zeneca UAE
- Neopharma
- Baxter A.G
- Medpharma
Major Pharmaceutical Industries in Asia:
- Pfizer
- GlaxoSmithKline
- Sanofi Aventis
- Merck
- Johnson and Johnson
- Amgen
- Novartis
- Roche
- Bristol-Myers Squibb
- Wyeth
- Eli Lilly
- Schering-Plough
- Abbott
- Takeda
- Boehringer Ingelheim
- Astellas
Major Pharmaceutical Industries Worldwide:
- Abbott Laboratories
- AbbVie
- Actavis
- Actelion
- Baxter International
- Biocon
- Biogen Idec
- Boehringer-Ingelheim
- Bristol-Myers Squibb
- Hetero Drugs
- Hoffmann–La Roche
- Intas Biopharmaceuticals
- Johnson & Johnson
- Lupin Limited
- Merck & Co.
- Mylan
- Novartis
- Novo Nordisk
- Procter & Gamble
- Purdue Pharma
- Sun Pharmaceutical
- Takeda Pharmaceutical Co.
- Teva Pharmaceuticals
- Torrent Pharmaceuticals
- Vertex Pharmaceuticals
- Wockhardt
Statistical Representation:
Target Audience:
The target audience will from the pharmaceutical, biotechnology industries, Contract manufacturing organizations, associations and societies related to Pharmaceutical field, Pharmaceutical research centres, students and professors from the academia of the various universities, researchers, scientists, experts, business delegates in the field of Pharmaceutical Sciences.
Pharmaceutical Technologies and Markets in Asia:
The Asia Pacific regional pharmaceutical market is expanding rapidly due to a rising, affluent, middle-class, and governments' push for healthcare reforms. Overall sales of prescription drugs and over-the-counter (OTC) medicines are forecast to increase from $276.6 billion in 2013 to $384.7 billion in 2018, representing a five-year compound annual growth (CAGR) of 7.0%. It is worth noting that by 2018, overall pharmaceutical sales in Asia Pacific is expected to exceed overall drug sales in Western Europe and the US combined.
Pharmaceutical Technologies and Markets worldwide:
Overall sales in the Pharmaceutical market amounted to $34.2 billionin 2007, and decreased slightly to $31.1 billion in 2009. By 2014, they are projected to reach $32.2 billion, for a 5-year compound annual growth rate (CAGR) of 0.7%.
The largest segment of the market, prescription drugs, was valued at $27.4 billion in 2008; this is expected to decrease to $26,3 billion in 2009, and to reach $27 billion in 2014, for a 5-year CAGR of 0.5%.The global excipients market was at $4.6 billion in 2010 and it is expected to reach at $4.9 billion in 2011. It is further anticipated to increase to $6.7 billion by 2016 at a compound annual growth rate (CAGR) of 6.5%.
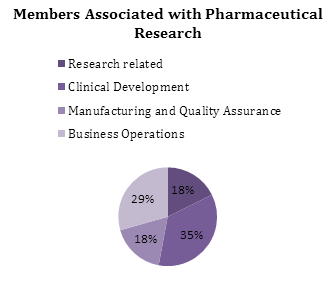
Members Associated with Pharmaceutical Research:-
Research Positions:-
- Lab technician
- Research Associate
- Research Scientist
Clinical Development and medical Jobs:-
- Clinical Research Physician
- Clinical Research Associate
- Regulatory Affairs Associate
- Bio Statistician
- Clinical Data Manager
- Medical Science Liaison
Manufacturing and Quality Assurance Jobs:-
- Process Engineer
- Quality Control Analyst
- Quality Assurance Specialist
Business Operations Jobs:-
- Market research Analyst
- Associate Product Manager
- Product Manager
- Strategy Director
- Business Development Manager
Conference Highlights
- Drug Targeting & Drug Development
- Pharmaceutics & Biopharmaceutics
- Pharmaceutical Research
- Pharmaceutical Materials
- Pharmaceutical Formulations
- Pharmaceutical Biotechnology
- Pharmaceutical Nanotechnology
- Pharmaceutical Technology
- Pharmaceutical Manufacturing
- Pharmaceutical Engineering
- Drug Delivery Technologies
- Medical Devices
- Drug Regulatory Affairs
- Pharmaceutical Management
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | April 24-26, 2017 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Pharmaceutica Analytica Acta
- Drug Designing: Open Access
- Research & Reviews in Pharmacy and Pharmaceutical Sciences
Abstracts will be provided with Digital Object Identifier by
































