Theme: Future Trends in the Development of Pharmaceuticals
Pharma Tech 2018


Dear colleagues and friends
On behalf of the organizing committee of the International Conference and Exhibition on Pharmaceutical Development and Technology and as keynote speakers, I invite you to our next international conference in Osaka, Japan, on May 11-12, 2018.
This global congress incorporates a scientific program by the global experts to explore the future aspect for next generation.
I invite you to participate and send your abstracts and put them to the consideration of our prestigious international scientific committee. Deadline May 07th, 2018 SUBMIT HERE
Main objectives of Pharmaceutical Development and Technology:
- Systemic, Holistic and Proactive approach to Pharmaceutical field.
- Emphasizes Product, Process understanding and process control.
- Develop a drug/medicine with appropriate quality, dosage form and strength.
- Designing mechanisms to target drugs to specific cells or tissues
- Developing delivery systems for slow release to maintain a level therapeutic dose.
- Developing novel technologies that can be used in specific conditions.
I await you in the beautiful city of Osaka, the cosmopolitan capital of Japan, one of the most visited cities in the world.
Sincerely Yours
Dr. Saeed Qureshi
Principal at PharmacoMechanics
Universiteit Gent
Ottawa, Canada
Keynote Speaker & Organizing Committee for International Conference and Exhibition on Pharmaceutical Development and Technology
Conference series LLC ltd., UK
Conference Series is a renowned organization that organizes highly notable pharmaceutical conferences and healthcare conferences throughout the globe. ConferenceSeries invites all the participants from all over the world to attend “2nd International Conference and Exhibition on Pharmaceutical Development and Technology” during May 11-12, 2018 in Osaka, Japan, which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
Pharma Tech 2018 is a latest technological platform to aid efficient drug discovery and development. These platforms range from vast chemical libraries, ultra-high throughput screening and huge genetic databases in discovery, to predictive toxicology platforms and even deep-seated knowledge of particular therapeutic areas in development. New research platforms designed to help its teams of investigators focus on a set of key strategic initiatives that will help guide their multibillion-dollar drug development program to advance new medicines that can get ahead of a disease and prevent it from taking a toll.
Pharmaceutical technology is a collective term for technologies to develop candidate compounds that have either been discovered or created into commercial pharmaceutical products. These products are made by transforming chemical compounds with useful effects on the human body into high-quality dosage forms that can appropriately exhibit effects against disease. Pharmaceutical technologies are divided into the following three functions.
Process technology for researching synthetic methods to be used to manufacture candidate compounds efficiently and consistently in large amounts and with high quality. Formulation technology for investigating dosage forms, formulations, and packages based on absorption stability, and usability in consideration of the characteristics of candidate compounds, and then selecting and preparing the optimal administration form. Analytical and quality evaluation technology for establishing a variety of analytical and quality evaluation systems to properly and appropriately assure the quality of the pharmaceutical products.
Efficacy and safety are the primary requirements for any pharmaceutical product. However, it is becoming significantly more important to provide pharmaceutical products that can be more easily used by patients, healthcare professionals, and caregivers in order to respond to the rapid aging of society and the needs for advanced medical care. Examples of user-friendly pharmaceutical products include extended-release tablets, which reduce the frequency at which pharmaceuticals must be administrated, and orally disintegrating (OD) tablets, which can be taken without water. Meanwhile, examples of innovation for healthcare professionals include syringes that are prefilled with drug solutions to reduce the hassle of preparation and the risk of needle injuries, as well as IC tags for pharmaceutical products or packages that are helpful in preventing medical errors. Additionally, we are utilizing various formulation technologies to provide user-friendly pharmaceutical products adding new value. Such innovations on this front include package designs and tablets with the product name printed on them in order to prevent any misuse.
We are advancing the development of new synthetic processes based on the eco-friendly concept of “green chemistry,” which is aimed at achieving global environmental sustainability through means such as preventing pollution and reducing consumption of materials and energy.
Why to attend???
With members from around the world focused on learning about innovations, latest technologies, different drug formulations for effective treatment and new drug moieties through pharmaceutical technology and its importance, this is your best opportunity to reach the largest assemblage of participants from the Clinical Research organizations, Pharmaceutical companies and technology related societies Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new research developments, and receive name recognition at this 3-day event. World-renowned speakers, the most recent issues, approaches, and the novel trends in Pharmaceutical Technology are hallmarks of this conference
Major sessions included at this conference are Pre-formulation studies, Drug Formulation Procedures, Drug Product Manufacturing, Pharmaceutical Engineering, Pharmaceutical Manufacturing, Pharmaceutical Technology, Technologies in Drug Delivery, Types of Pharmaceutical Formulations, Regulatory Requirements for Pharmaceuticals, Thermodynamics, Pharmaceutical Management.
Conference Keywords
- Pharmaceutical Industry:
- Pharmaceutical Products:
- Pharmaceutical:
- Pharmaceutical Technology:
- Pharmaceutical Production:
- Pharmaceutical Engineering:
- Drug Research:
- Pharmaceutical Research:
- Pharmaceutical Marketing:
- Pharmaceutics:
- Drug development:
- Global Pharmaceutical Market:
- Pharma Marketing strategies:
- Regulatory Affairs:
- Medical Representative:
- Innovations in drug delivery:
- Drug Delivery Technology:
- Pharmaceutical Chemistry:
- Radio Pharmaceuticals:
- Pharmaceutical services:
- Pharmaceutical Manufacturers:
- Prescription Drugs:
- Bio Informatics:
- Pharmaceutical Nanotechnology:
- Bio pharmaceutics:
- Pharmaceutical Active Ingredient:
- Clinical Trial:
- Medical Device:
- Pharmaceutical Journal:
- Pharmaceutical Laboratory:
- Drug Distribution:
- Cosmeceutical:
- Pharmaceutical Logistics:
- Pharmaceutical Analysis:
- Pharmaceutical Microbiology:
- Pharmaceutical Compounding:
- Pharmaceutical Grade:
- Healthcare Professionals:
- Pharmaceutical Formulation:
- Pharmaceutical Executive:
- Pharmaceutical Patent:
- Pharmaceutical Excipients:
- Pharmaceutical Advertising:
- Pharmaceutical Testing:
- Pharmaceutical Law:
- Finished Product:
- Pharmaceutical Processing:
- Pharmaceutical Innovations:
- Pharmaceutical Quality Assurance:
- Pharmaceutical Intermediates:
The terminology Pharmaceutical Industry analyze, establish ,discovers, produces, manufactures, develops, and markets the pharmaceutical drugs for use as medicines. These companies usually arrangement with generic or brand medicines and medical devices.
This includes Pharmaceutical Technology, Pharmaceutical Products, Quality Control, Good manufacturing Practices.
The global Pharmaceutical market is expected to reach USD 28.66 Billion by 2022 at a CAGR of 15.8%.
Pharmaceutical products are usually named as medicines or drugs that are a vital component of all ancient, traditional medicine. It is important for those products to be safe, effective, of good quality, and must be prescribed and used rationally.
This includes Pharmaceutical materials, Pharmaceutical formulations, and Pharmaceutical excipients.
The global Pharma Materials market to increase to $ 6.78 billion in 2021 at a compound annual growth rate (CAGR) of 8.41% for 2016-2021.
A pharmaceutical is a medicament which is used to prevent, identify and cure a disease. Drug treatment is an essential section of the medical field and depends on the science of pharmacology for the advancement and pharmacy for proper management.
This includes Pharmaceutical products, Pharmaceutical manufacturing, Drug targeting and drug delivery.
Pharmaceutical Technology is the higher origin of reviewed scientific scrutiny and analyses for scientists, engineers, and managers involved in the identification, evolution, manufacturing, formulation and drug delivery, API composition, analytical technology and trial, packaging, IT, outsourcing, and regulatory performance in the pharmaceutical and biotechnology industries.
This includes Pharmaceutical Biotechnology, Pharmaceutical Nanotechnology, Drug delivery systems.
The global market Pharmaceutical technology market is expected to reach USD 49.27 Billion in 2025, at a CAGR of 7.5%
Pharmaceutical production is the Forward of industrial-scale synthesis of pharmaceutical medicate by pharmaceutical Unit. The Manufacturing of drug can be disconnected down into a sequence of unit operations, such as Formulation and pre-formulation development, Powder blending, Milling, Granulation, Hot melt extrusion and others.
This includes Pharmaceutical manufacturing, pharmaceutical products, pharmaceutical materials and pharmaceutical formulations.
Pharmaceutical engineering is a term from the department of pharmaceutical science and technology that covers manufacturing products, and their development ,procedure, and components in the pharmaceuticals companies (i.e. medicines & biologics).
This includes Pharmaceutical technology, Pharmaceutical development, and Novel drug delivery systems.
The global market for pharmaceutical manufacturing should reach $ 679.9 Million by 2021 at a compound annual growth rate (CAGR) of 10.1% from 2016 to 2021
In the branches of medicine, pharmaceutical technology and pharmacology, drug research is the innovative method by which brand new candidate medications are discovered. Initially, these were discovered by identifying the active constituent from traditional remedies or by serendipitous discovery.
This includes Pharmaceutical research, Drug targeting and drug delivery.
The global Drug targeting and Drug Development market should reach $401.1 Million by 2020, growing at a compound annual growth rate (CAGR) of 6.65%from 2015 to 2020.
Medical Experts are continuously finding the innovative and the better ways of treating illness or disease. Pharmaceutical Research includes the study about what links the medical research to a medicine for becoming available to physicians and patients.
This includes Pharmaceutical technology, Pharmaceutical development, Drug Delivery systems.
The global Pharmaceutical research market for advanced Techniques is Certain to reach USD $12.60 billion by 2020, a five-year compound annual growth rate (CAGR) of 6.2% through 2020. According to Present report by Grand View Research
Pharmaceutical marketing is a term which is also named as medico-marketing or Pharma marketing in some divisions, is basically a method of advertising and promoting the demand for pharmaceutical drugs.
This includes Pharmaceutical products, pharmaceutical materials, Pharmaceutical Industry, Pharmaceutical manufacturing.
The global Pharmaceutical market is expected to reach USD 28.66 Billion by 2022 at a CAGR of 15.8%.
Pharmaceutics is the branch of pharmacy that involves the process of modifying a brand new chemical substance into a drug that can be used safely and effectively by patients. It is also named as the science of dosage form design.
This includes Pharmaceutical formulations, Pharmaceutical safety, Quality control and Biopharmaceutical.
The global Biopharmaceutical market for advanced Techniques is Certain to reach USD $ 4.71 billion by 2020, a five-year compound annual growth rate (CAGR) of 9.5% through 2020. According to Present report by Grand View Research.
Drug development is the method of getting a totally novel pharmaceutical entity into the pharmaceutical market after a strong compound has been identified by the method of drug discovery.
This includes drug discovery, drug delivery, drug targeting and drug delivery technologies.
The global Drug targeting and Drug Development market should reach $401.1 Million by 2020, growing at a compound annual growth rate (CAGR) of 6.65%from 2015 to 2020.
Global Pharmaceutical Industry defines that the pharmaceutical industry is important for the evolution, manufacturing and marketing of drugs. Hence its high importance as a global sector is inarguable.
This includes pharmaceutical marketing, Pharmaceutical industry, Pharmaceutical manufacturing.
The global Pharmaceutical market is expected to reach USD 28.66 Billion by 2022 at a CAGR of 15.8%.
Pharmaceutical marketing strategy must be evolved once identifying and researching many factors of the industry. Marketing is usually an option to get your brand out to create awareness about the presence of the company and to influence maximum of the customers to choose you over competing brands.
This includes pharmaceutical industry, Pharmaceutical technology, pharmaceutical marketing.
Regulatory affairs is totally a new profession which is evolved from the need of the governments to protect the public health through controlling the safety and efficacy of substances in the areas including pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines.
This includes drug regulatory affairs, pharmaceutical law, and pharmaceutical safety.
The global regulatory affairs market is Certain to reach USD $401.1 million by 2020, a five-year compound annual growth rate (CAGR) of 6.65% through 2020.
Medical representatives are the main link between the pharmaceutical industries and the health care professionals. Excellent sales skills are the important requirement to be best medical representative.
This includes pharmaceutical marketing, pharmaceutical products, pharmaceutical industry, pharmaceutical advertising.
Recent advancement taking place in the field of drug delivery system with respected to the change in external environment and the habitat is called as Innovations in drug delivery system.
This includes pharmaceutical technology, drug delivery technology, pharmaceutical technology, Drug discovery.
The global Pharmaceutical research market for advanced Techniques is Certain to reach USD $12.60 billion by 2020, a five-year compound annual growth rate (CAGR) of 6.2% through 2020. According to Present report by Grand View Research
Drug delivery technology increases the absorption of the drug, its efficacy, and patient compliance. Taste maskers increase the commercial viability of your pharmaceutical products by neutralizing the strong, bitter tastes of certain oral medical formulations.
This includes nanotechnology, Nano delivery, Liposomes, drug targeting.
The global Drug targeting and Drug Development market should reach $401.1 Million by 2020, growing at a compound annual growth rate (CAGR) of 6.65%from 2015 to 2020.
Pharmaceutical chemistry is the study of drugs, and it involves drug development. This includes drug discovery, delivery, absorption, metabolism, and more. There are elements of biomedical analysis, pharmacology, pharmacokinetics, and pharmacodynamics. Pharmaceutical chemistry work is usually done in a lab setting.
This includes pharmaceutical technology, drug development, drug delivery.
Radiopharmaceuticals are unique medicinal formulations containing Radioisotopes Radiopharmaceuticals can be used as diagnostic and therapeutic agents.
This is pharmaceuticals, pharmaceutical formulations, pharmaceutical complications.
Pharmaceutical service Those services which relate to the supply of drugs, medicines and appliances prescribed world health Organization .
This includes pharmaceutical care, pharmacy practice, community pharmacy.
Pharmaceutical manufacturing is the process of industrial-scale synthesis of pharmaceutical drugs by pharmaceutical companies. The process of drug manufacturing can be broken down into a series of unit operations, such as milling, granulation, coating, tablet pressing, and others. This includes Pharmaceutical Nanotechnology, Pharmaceutical Biotechnology, Pharmaceutical Formulations, Good manufacturing Practices
The global market for pharmaceutical manufacturing should reach $ 4,882.9 Million by 2021 at a compound annual growth rate (CAGR) of 52.42% from 2016 to 2021
A prescription drug is a pharmaceutical drug that legally requires a medical prescription to be dispensed, Prescription drugs are the third most commonly abused category of drugs, behind alcohol and marijuana and ahead of cocaine, heroin, and methamphetamine. This includes Pharmaceutical Research, Pharmaceutical Materials, Drug Targeting & Drug Development, Good manufacturing Practices
Bioinformatics is a multidisciplinary field that advance approach and software tools for understanding biological data. As an multidisciplinary field of science, bioinformatics combines computer science, statistics, mathematics, and engineering to analyze and establish biological knowledge.
This includes Pharmaceutics & Bio pharmaceutics, Pharmaceutical Biotechnology, Pharmaceutical Formulations, Good manufacturing Practices
Pharmaceutical Nanotechnology:
Pharmaceutical nanotechnology grasp function of Nano science to pharmacy as nanomaterial, and as equipment like drug delivery, diagnostic, imaging and biosensor. Nano medicine is explain as submicron size (<1um) modules, applicationfortreatment, diagnosis, monitoring, and control of biological system.
This includes Pharmaceutical Technology, Pharmaceutical Nanotechnology, Pharmaceutical Formulations, Good manufacturing Practices
The global Pharma Nanotechnology market is expected to reach USD 50 Billion in 2025, at a CAGR of 11-12%
Pharmaceutical Biotechnology further developed as an established biological drug product which includes all the pharmaceuticals and Medical products produced and extracted from, or semi synthesized from biological origin This includes Pharmaceutical Biotechnology, Bio pharmaceutics, Pharmaceutical Formulations, Good manufacturing Practices
The global Biopharmaceutical market for advanced Techniques is certain to reach USD $ 4.71 billion by 2020, a five-year compound annual growth rate (CAGR) of 9.5% through 2020. According to Present report by Grand View Research
Pharmaceutical Active Ingredient:
An active ingredient (AI) is the ingredient in a pharmaceutical drug that is biologically active. The similar terms active pharmaceutical ingredient(API) and bulk active are also used in medicine, and the term active substance may be used for natural products.
This includes Drug Regulatory Affairs, Pharmaceutical Technology, Pharmaceutical Formulations, Good manufacturing Practices
Clinical trials are studies about the research work that confirms if a medical idea is effective and safe for the use of humans. This studies also shows which medical way is the best for a particular group of illnesses or people. They produce the best data available for health care decisionmaking.
This includes Medical Devices, Pharmaceutical Technology, Pharmaceutical Formulations, Good manufacturing Practices
Medical Devices are used on humans that have therapeutic benefits which generally have a physical or mechanical effect on the body or are used to measure or monitor functions of the body
This includes Medical Devices, Pharmaceutical Management, Pharmaceutical Research, Good manufacturing Practices
The global Medical devices Market is projected to reach USD 1.88 Billion by 2022 from USD 0.84 Billion in 2017, at a CAGR of 17.5% during the forecast period
The Pharmaceutical Journal is a professional journal covering various aspects of pharmacy, including pharmacology and pharmaceutics. It is published by, and is the official journal of, Britain's professional organization for pharmacists, the Royal Pharmaceutical Society
This includes Drug Regulatory Affairs, Pharmaceutical Management, Pharmaceutical Research, Good manufacturing Practices
The Pharmaceutical Laboratory provides both analytical and investigative services mainly to the Health Products Regulation Group on the regulatory testing of pharmaceutical and health products under the Health Products Act, Medicines Act and Poisons Act.
This includes Pharmaceutical Nanotechnology, Pharmaceutical Biotechnology, Pharmaceutical Formulations, Good manufacturing Practices
A Centralized unit-dose drug distribution system(CUDD): q All in-patient drugs are dispensed in unit doses and all the drugs are stored in central area of the pharmacy and dispensed at the time the dose is due to be given to the patient.
This includes drug absorption, bioavailability, and pharmacokinetics.
Cosmeceuticals is the combination of cosmetics and pharmaceuticals. Cosmeceuticals are cosmetic products with bioactive ingredients purported to have medical or drug-like benefits.
This includes pharmaceuticals, cosmeceutics, cosmetics, beauty products.
Developing warehousing structures is a major focus area for the pharmaceutical industry and outsourcing to logistics service providers (LSPs) is on the increase. Cost reduction and greater visibility in the supply chain seem to be the main advantages of outsourcing logistics services.
This includes pharmaceutical marketing, pharmaceutical industry, pharmaceutical manufacturing, good manufacturing practices.
Pharmaceutical analysis is a branch of practical chemistry that involves a series of process for identification, determination, quantification and purification of a substance, separation of the components of a solution or mixture, or determination of structure of chemical compounds.
This includes pharmaceutical chemistry, chemical analysis, Organic chemistry, Inorganic chemistry, medicinal chemistry.
Drug safety is a major focus of pharmaceutical microbiology. Pathogenic bacteria, fungi (yeasts) and toxins produced by microorganisms are all possible contaminants of medicines- although stringent, regulated processes are in place to ensure the risk is minimal.
This includes bacteria, microorganisms, infections, disinfectants, sterilization.
Pharmacy compounding is the art and science of preparing personalized medications for patients. Compounded medications are made based on a practitioner’s prescription in which individual ingredients are mixed together in the exact strength and dosage form required by the patient.
This includes pharmacy, pharmacy practice, pharmaceutical dispensing, and prescription.
The difference between the grades is one of how much of these other substances are present in the product. There are several criteria by which pharmaceutical grade are judged. The product must be in excess of 99% purity with no binders, fillers, excipients, dyes, or unknown substances. United States Pharmacopeia (USP).
This includes pharmacopeia, pharmaceutical standards, pharmaceutical references.
A health professional, health practitioner or healthcare provider is an individual who provides preventive, curative, promotional or rehabilitative health care services in a systematic way to people, families or communities.
This includes pharmaceutical services, pharmaceutical care.
Pharmaceutical formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form.
This includes pharmaceutical products, pharmaceuticals, pharmaceutical dosage form, and dose.
The global Pharmaceutical Formulations market is estimated to reach USD 8.24 Billion by 2021, growing at a CAGR of 6.8% during the forecast period
Pharmaceutical Executive Global Direct is delivered directly to your inbox every fourth Tuesday and combines the latest and best global articles from the industry-leading Pharmaceutical Executive magazine with up-to-the-minute European news and features.
This includes Marketing authorization, Intellectual Property rights, Clinical Affairs & Regulatory Strategies.
A generic drug is a pharmaceutical drug that is equivalent to a brand-name product in dosage, strength, route of administration, quality, performance and intended use. In most cases, generic products become available after the patent protections afforded to a drug's original developer expire.
This includes Nano Drug delivery, Nano Pharmaceuticals, Nano Liposomes, and Nano medicine
Pharmaceutical excipients are substances other than the pharmacologically active drug or pro drug which are included in the manufacturing process or are contained in a finished pharmaceutical product dosage form.
This includes Pharmaceutical validation, Freeze drying Technique, Pharmaceutical Process Equipment.
sometimes called medico-marketing or Pharma marketing in some countries is the business of advertising or otherwise promoting the sale of pharmaceutical drugs. Many countries have measures in place to limit advertising by pharmaceutical companies.
This includes Pharmaceutical Packaging and Labelling, Biomedical Instrumentation Measurements, Pharmaceutical Sales.
The global Pharmaceutical market is expected to reach USD 28.66 Billion by 2022 at a CAGR of 15.8%.
Pharmaceutical Testing. Adpen Laboratories is the standard for quality and excellence and our knowledge and experience enable us to provide assistance to the ongoing operations of pharmaceutical companies, medical device manufacturers and research universities.
This includes Ophthalmic Formulation, Rational Drug Design, Quality assurence.
Another important area of pharmaceutical law is in product safety and marketing. Pharmaceuticals are among the most highly regulated products in the U.S., and must pass stringent testing by organizations such as the Food and Drug Administration (FDA) before they are even allowed onto the market.
This includes Development of Vaccines, Small Drug Molecules, Gene specific drugs.
A good purchased as a "raw material" goes into the manufacture of a product. A good only partially completed during the manufacturing process is called "work in process". When the good is completed as to manufacturing but not yet sold or distributed to the end-user, it is called a "finished good".This includes Pharmaceutical Excipients, Types of Drug Formulation, Bioprocessing.
Drug manufacturing is the process of industrial-scale synthesis of pharmaceutical drugs by pharmaceutical companies. The process of drug manufacturing can be broken down into a series of unit operations, such as milling, granulation, coating, tablet pressing, and others.
This includes Biomaterials, Polymers, Graphene, Quantum Dots.
Pharmaceutical innovations are currently guided by a patent system; the patent system protects the innovator of medicines for a period of time. The patent system does not currently stimulate innovation or pricing that provides access to medicine for those who need it the most, it provides for profitable innovation.
This includes Positron-Emmi son Tomographic (PET) Scanning, Computed Tomographic Scanning (CT Scanning), Pacemakers and Defibrillators.
Pharmaceutical Quality Assurance:
Medicines quality assurance. Quality assurance is a wide-ranging concept covering all matters that individually or collectively influence the quality of a product. With regard to pharmaceuticals, quality assurance can be divided into major areas: development, quality control, production, distribution, and inspections.
This includes Forensic devices, 3D printing of medical devices, Regulatory requirements of pharmaceutical products.
The Pharmaceutical Ingredients segment has two product groups: Pharmaceutical Intermediates and Active Pharmaceutical Ingredients (APIs).
This includes Pharmaceutical Management, Pharmaceutical Industries, Food and Drug administration.
Track 1. Challanges in Pharmaceutical Formulations
This track includes Pharmaceutical Formulation which is the process in which different chemical substances i.e., active chemical substances will combined together to produce a medical compound i.e., medical drug. This process involves production of drug which characterized by two things: Stability of the product, second Acceptability to the patient. Formulation studies focuses on factors like particle size, polymorphism, pH and solubility, in order to check whether these factors will effect on bioavailability of the drug or not. Pharmaceutical Formulations include Ophthalmic Formulation, Paediatric Formulation Development, Topical Formulation and Medication Formulation.
This track is representing Pharmaceutical Excipients Pharmaceutical Product Development ,Types of Drug Formulation ,Ophthalmic Formulation ,Pediatric Formulation Development, Medication Formulation ,Topical Formulations
Pharmaceutical Formulations Market Size worth $ 8.24 Billion By 2021 | CAGR: 6.8%
The global Pharmaceutical Formulations market is estimated to reach USD 8.24 Billion by 2021, growing at a CAGR of 6.8% during the forecast period
Related Conferences:
12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress during February 26-27, 2018 at London, UK; 11th European Biosimilars Congress during April 26-27, 2018 at Rome, Italy; 14th Nanomedicine and Pharmaceutical Nanotechnology Conference during April 09-11, 2018 at Amsterdam, Netherlands; 10th Pharmacology Congress during Jul 30-Aug 01, 2018 at Barcelona, Spain.
Nasal Drug Delivery Conference during April 12-13, 2018 at London, UK; 9th Drug Delivery and formulation Summitduring March 12-18, 2018 at Berlin, Germany; 3rd European Pharmaceutics Conference during April 1- 2, 2019 at Bologna, Italy; 3rd Nanomedicine, Drug Delivery, and Tissue Engineering Conference during April 10, 2018 at Budapest, Hungary.
Related Societies:
American Association of Pharmaceutical Scientists (AAPS), Pharmaceutical Society of New Zealand Incorporated (PSNZI), Pharmaceutical & Bioscience Society (PBS), Royal Pharmaceutical Society (RPS), Bangladesh Pharmaceutical Society (BPS), Society for Pharmaceutical Dissolution Science (SPDC)
Track 2. Advancements in Drug Targeting and Drug Development
Noticing drug targets plays essential roles in designing noval drugs and combating diseases. Unfortunately, our current knowledge about drug targets is far from comprehensive. Screening drug targets in the lab is an expensive and tedious procedure. In the past decade, the accumulation of various types of study of science related data makes it possible to develop computational approaches to predict drug targets.
This session represents Rational drug design Biotargets Computer Aided drug Design Bioweapons Tumor Targeting Mitochondrial Targeting
Drug Targeting and Drug Development Market Size worth $ 401.1 Million By 2020 | CAGR: 6.65%
The global Drug targeting and Drug Development market should reach $401.1 Million by 2020, growing at a compound annual growth rate (CAGR) of 6.65%from 2015 to 2020.
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related Societies:
Vancouver Pharmaceutical & Bioscience Society(VPBS), Chinese American Biopharmaceutical Society(CABPS), Pharmacy Society of Wisconsin(PSW), Pharmaceutical Society of Ghana(PSG), Academy Of Pharmaceutical Sciences(APS), Association of British Pharmaceutical Industry(ABPI), Pharmaceutical and Health Care Sciences Society(PHCSS), Royal Society of Chemistry(RSC), International Society for Pharmaceutical Engineering(ISPE)
Track 3. Scope of Pharmaceutical Research
In the third track of ‘Pharmatech’ will be discussing about the Pharmaceutical Research that covers research spanning the entire spectrum of drug discovery, development, evaluation, and regulatory approval. Small drug molecules, biotechnology products which also includes genes, peptides, proteins and vaccines, and genetically engineered cells are an integral part of recent published papers.
This track includes the study about Small Drug Molecules, Development of Vaccines, Gene specific Drugs, Proteins and Peptides.
Pharmaceutical Research Market Size worth $ 12.60 Billion By 2020 | CAGR: 6.2%
The global Pharmaceutical research market for advanced Techniques is Certain to reach USD $12.60 billion by 2020, a five-year compound annual growth rate (CAGR) of 6.2% through 2020. According to Present report by Grand View Research
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related Societies:
European Society of Biochemical Pharmacology (ESBP); European Society of Ethnopharmacology (ESE); French Society of Pharmacology (SFP); German Society for Experimental and Clinical Pharmacology and Toxicology (DGPT); Indian Pharmacological Society (IPS); Italian Society of Pharmacology (SIF); Japanese Pharmacological Society(JPS); Korean Society of Applied Pharmacology (KSAP); Korean Society of Pharmacology (KOSPHAR); Association for Ocular Pharmacology and Therapeutics (AOPT); British Association for Psychopharmacology (BAP)
Track 4. Recent Development of Pharmaceutical Materials
This session is of Pharmaceutical materials which are substances that are used in medical devices or in contact with biological systems. The Quality control and standardization of raw materials in pharmaceuticals has been a major step in the pre formulation studies of API’s, hence many recent pharmaceutical research organization have taken a step to overcome these aspects by thorough quality control techniques. Biomaterials use impression from medicine, biology, chemistry, materials science and engineering. Although biomaterials are primarily used for medical applications, they are also used to multiply cells in culture, to assay for blood proteins in the clinical laboratory, in proceeding biomolecules in biotechnology, for the regulation of fertility of implants in cattle and in diagnostic gene arrays and as well as in the adequacy of oysters and for investigational cell-silicon "biochips." The commonality of these applications is the interaction between biological systems and simulated or modified natural materials.
This term is represents the materials like Biomaterials Exfoliated Vermiculite Polymers Graphene Quantum Dots Microcapsules Hybrid materials
Pharma Materials Market Size worth $ 6.78 Billion By 2021 | CAGR: 8.41%
The global Pharma Materials market to increase to $ 6.78 billion in 2021 at a compound annual growth rate (CAGR) of 8.41% for 2016-2021.
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track 5 : Recent Developments in Pharmaceutics and Bio pharmaceutics.
This session of Pharmaceutics and Bio pharmaceutics concerned with all Advancement and Development of Pharmaceutics and Bio pharmaceutics. Bio pharmaceutics examines the inter relationship of the physical/chemical properties of the drug, the dosage form (drug product) in which the drug is given, and the route of administration on the rate and extent of systemic drug absorption. Pharmaceutics concerns with manufacture of drugs vaccines biological medical devices equipment and their design and validation. This also includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials.
This session is represents with Physical pharmacy, Pro-drug design, Biologics, Pharmaceutical Analysis, Pharmaceutical Packaging, Drug Pharmacokinetics, Quality control, Good Manufacturing Practices
Biopharmaceutical Market Size worth $ 4.71 Billion By 2020 | CAGR: 9.5%
The global Biopharmaceutical market for advanced Techniques is Certain to reach USD $ 4.71 billion by 2020, a five-year compound annual growth rate (CAGR) of 9.5% through 2020. According to Present report by Grand View Research.
Related conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related societies:
Austrian Pharmaceutical Society, Canadian Society for Pharmaceutical Sciences, Drug Information Association, Georgia Pharmacy Association, Illinois Pharmacists Association, International Pharmaceutical Federation (FIP).
Track 6: Advancements in Pharmaceutical Biotechnology
The term is Pharmaceutical Biotechnology which is the branch of science that deals with all the technologies required for manufacturing, producing and registration of biological drugs. Pharmaceutical Biotechnology is an increasingly essential area of science and technology. The Pharmaceutical Biotechnology is largely spread area , ranging from many ethical issues to changes in healthcare practices and a significant contribution to the development of national economy. Biotech drug makers mainly use those microorganisms or highly complex proteins from genetically-modified living cells as components in medications to treat various diseases and conditions, from cancer to rheumatoid arthritis to multiple sclerosis.
This session includes Microarray Technology, Industrial Biotechnology ,Bio formulations, Vaccines and Antibiotics ,Nucleic Acid Products ,Biologically inspired Pharmaceutics
Pharmaceutical Bio technology Market worth $ 49.27 Billion By 2019 | CAGR: 7.5%
The global market Pharmaceutical Bio technology market is expected to reach USD 49.27 Billion in 2025, at a CAGR of 7.5%
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related Societies:
Austrian Association of Molecular Life Sciences and Biotechnology; Austrian Society for Hygiene, Microbiology and Preventive Medicine; Association for General and Applied Microbiology; French Society for Microbiology; Italian Society of Agro-Food and Microbial Biotechnologies; Federation of European Microbiological Societies (FEMS);European Molecular Biology Organization (EMBO);Society for Applied Microbiology (SfAM);Swiss Society for Infectious Disease American Association of pharmaceutical Scientists; The Canadian Society of Microbiologists;American Academy of Microbiology; International Union of Microbiological Societies; Anaerobe Society of the Americas, Inc; Infectious Diseases Society of America Asia Pacific Society for Marine Biotechnology, Committee of Asia Pacific Electron Microscopy Societies, Federation of Asia Pacific Microbiological Societies, International Society for Applied Phycology.
Track 7: Approaches in Pharmaceutical Nanotechnology
This term Nano pharmaceuticals gives an ability to identify the diseases at very earlier stages and the diagnostic applications can build upon the conventional procedures using nanoparticles. Nano pharmaceuticals is considered as an emerging field where the sizes of the drug molecule or a therapeutic delivery system works at a Nano scale. In the pharmaceutical industry, a never ending issue is the difficulty of delivering the appropriate dose of a particular active medication to specific diseased site. Nano pharmaceuticals have enormous potential in addressing this failure of traditional therapeutics which offers site-specific targeting of active agents.
This track is representing Biomedical Nanotechnology Nano technology and Nano medicine Nano Drug Delivery Nano Liposomes Nano Pharmaceuticals Applied Nanotechnology
Pharma Nanotechnology Market Size worth $50 Billion By 2022 | CAGR: 11-12%
The global Pharma Nanotechnology market is expected to reach USD 50 Billion in 2025, at a CAGR of 11-12%
Related Conferences:
12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress during February 26-27, 2018 at London, UK; 23rd Nanomaterials and Nanotechnology Conference during March 15-16, 2018 at London, UK; 14th Nanomedicine and Pharmaceutical Nanotechnology Conference during April 09-11, 2018 at Amsterdam, Netherlands; 11th European Biosimilars Congress during April 26-27, 2018 at Rome, Italy; 24th Nano Conference during May 07-08, 2018 at Rome, Italy.
20th Nanotechnology and Nanomedicine Conference during July 09, 2018 at Prague, Czech Republic; Pharma Conference and Expo during May 2-4, 2018 at Rome, Italy; 15th Nanosciences and Nanotechnologies conferenceduring July 03-06, 2018 at Greece
Related Societies:
The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Track 8. Innovations in Pharmaceutical Technology
This session is of Pharmaceutical technology which is the application of scientific knowledge or technology to pharmacy, pharmacology, and the pharmaceutical industry. It describes techniques , designs, and instrumentation in the preparation, compounding, manufacturing, packaging, dispensing and accumulating of narcotic and other preparations used in diagnostic and determinative procedures and in the treatment of patients.
This track represents Technology Transfer Pharmaceutical Technology Assessment Quality assurance Bioinformatics and Computational Biology Pharmacological Technology advances Pharmaceutical Packaging and labeling
Pharmaceutical technology Market worth $ 49.27 Billion By 2019 | CAGR: 7.5%
The global market Pharmaceutical technology market is expected to reach USD 49.27 Billion in 2025, at a CAGR of 7.5%
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related Societies:
Controlled Release Society (CRS) Institute of Pharmacy Management International (IPMI) International Academy of Compounding Pharmacists (IACP) International Association for Pharmaceutical Technology (APV) International eHealth Association International Federation of Pharmaceutical Manufacturers Associations (IFPMA) International Federation of Pharmaceutical Wholesalers (IFPW) International Pharmaceutical Excipients Council (IPEC) International Pharmaceutical Federation (FIP) International Pharmaceutical Students Federation (IPSF) International Society for Medical Publication Professionals (ISMPP) International Society of Oncology Pharmacy Practitioners (ISOPP) International Society for Pharmaceutical Engineering (ISPE) International Society for Pharmacoepidemiology (ISPE) International Society for the History of Pharmacy (ISHP)International Society for the Study of Xenobiotics (ISS) Federation of Asian Pharmaceutical Associations (FAPA) Asia Partnership Conference of Pharmaceutical Associations(APAC)
Track 9. Advancements in Pharmaceutical Manufacturing
In this track of ‘Pharmatech’ will be discussing about Drug manufacturing (Pharmaceutical Manufacturing)which is a process of industrial-scale manufacture of pharmaceutical components by pharmaceutical companies. The process of pharmaceutical manufacturing can be broken down into a various steps of unit operations, such as granulation, coating, milling, tablet compressing, and others. Start-up Pharmaceutical Companies, drug manufactures, contract pharmaceutical companies, pharmaceutical marketing agencies all play a major role in drug manufacture from its raw material to the final finished product.
This track is representing Active Pharmaceutical Ingredient synthesis Processing and Engineering Medication management Bioprocessing Molecular isotopic engineering Supply chain safety
Pharma Manufacturing Market Size worth $ 4,882.9 Million By 2021 | CAGR: 52.42%
The global market for pharmaceutical manufacturing should reach $ 4,882.9 Million by 2021 at a compound annual growth rate (CAGR) of 52.42% from 2016 to 2021
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related societies:
Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Track 10. Development of Pharmaceutical Engineering
The term Pharmaceutical Engineering is a branch of science and technology that mainly involves the pharmaceutical science that deals with the development and manufacturing of components, products, and processes in the pharmaceutical industry. While developing a drug involves several interrelated disciplines, the particular subfield of "pharmaceutical engineering" has emerged recently as a distinct engineering discipline. This now helps in solving the problems by bringing the principles and quantitative training of engineering to complement the other scientific fields that are already involved in drug development.
This term represents various engineering principles like Pre formulation Freeze Drying techniques Pharmaceutical sales Pharmaceutical Process Equipment GMP International standards Pharmaceutical validation
Pharma engineering Market Size worth $240.3 Billion By 2022 | CAGR: 11.8%
The global market for pharmaceutical manufacturing should reach $ 679.9 Million by 2021 at a compound annual growth rate (CAGR) of 10.1% from 2016 to 2021
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related societies:
American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP), Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC). Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI), Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS). Korean Research-based Pharmaceutical Industry Association (KRPIA), The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Kuwait Pharmaceutical Association (KPA).
Track 11. Innovations in Drug Delivery Technologies
This term Drug Delivery Technology is one of the unprecedented progresses of pharmaceutical nanotechnology during the past few decades, the conventional drug delivery systems (DDSs) have been involved into smart DDSs with stimuli-responsive characteristics. To increase their therapeutic efficacy and reduce the related side effects, active drug molecules should selectively accumulate in the diseased site for a prolonged duration with high controllability. In comparison to the conventional DDSs, the smart controlled DDSs can effectively reduce the dose frequency, while maintaining the drug concentration in targeted organs/tissues for a longer duration. In this case, the controlled DDSs provide wide insights and fascinating properties for decreasing the drug concentration fluctuation, reducing drug toxicities and improving therapeutic efficacy.
Drug Delivery Technologies Market Size worth $5.99Billion by 2022 | CAGR:8.2%
The Thermal analysis market is projected to reach USD 125.88 Billion by 2022, at a CAGR of 6.4% from 2017 to 2022.
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related societies:
American Association of Pharmaceutical Scientists(AAPS), Pharmaceutical Society of New Zealand Incorporated(PSNZI), Pharmaceutical & Bioscience Society(PBS), Royal Pharmaceutical Society(RPS), Bangladesh Pharmaceutical Society(BPS), Society for Pharmaceutical Dissolution Science(SPDC), Vancouver Pharmaceutical & Bioscience Society(VPBS), Chinese AmericanBiopharmaceutical Society(CABPS), Pharmacy Society of Wisconsin(PSW)
Track 12. Evolving of Medical Devices
In this track of “Pharmatech” we discuss about Instruments , apparatus, tools , software, material or other article, whether used alone or in combination used specifically for diagnostic or for therapeutic purposes and necessary for its proper application, intended for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease are medical devices.
The term medical devices includes Biomedical Instrumentation Measurements 3D printing of medical devices Instrumentation for Psychophysiological Measurements Instrumentation for the Experimental Analysis of Behavior Pacemakers and Defibrillators Ophthalmic and ENT Instruments Computed Tomographic Scanning (CT Scanning) Positron-Emission Tomographic (PET) Scanning Thermography and Tomography Elastography and Ultrasonography Respiratory Therapy Equipment Instrumentation for the Medical Use of Radioisotopes Forensic Devices
Medical devices Market Size worth $ 1.88 Billion by 2022 | CAGR: 17.5%
The global Medical devices Market is projected to reach USD 1.88 Billion by 2022 from USD 0.84 Billion in 2017, at a CAGR of 17.5% during the forecast period
Related Conferences:
Global Pharmacovigilance 2018, July 09-10, 2018 Sydney, Australia 5th Asia-Pacific Pharma Congress July 16-18, 2018 Melbourne, Australia 2nd International Conference and Exhibition on Nanomedicine and Drug Delivery May 14-16, 2018 Tokyo, Japan 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology April 09-11, 2018 Amsterdam, Netherlands 20th International Conference on Pharmacy and Pharmaceutical Sciences London, United Kingdom February 15 - 16, 2018 IIIrd International Conference on Researches in Science & Technology 20-21 Feb 2018, Dubai
Related societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track 13. Advancements of Drug Regulatory Affairs
The term Regulatory affairs is a profession within regulated industries. Regulatory affairs have a very specific meaning within the healthcare companies. Regulatory affairs is a new profession which is developed from the desire of governments to protect the public health by controlling the efficacy and nature of the products in the fields such as pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines. Regulatory Affairs is involved in the development of new medicinal products from early on, by integrating regulatory principles and by preparing and submitting the relevant regulatory dossiers to health authorities.Regulatory Affairs is involved in every step of the development of a noval medicine and in the post-marketing activities with medicinal products.
This track is representing Clinical Trials & Pharmacovigilance Regulatory compliance Regulatory requirements of pharmaceutical products Marketing Authorizations Clinical Affairs & Regulatory Strategies Intellectual Property Rights
Regulatory affairs Market Size worth $ 401.1 Million By 2020 | CAGR: 6.65%
The global regulatory affairs market is Certain to reach USD $401.1 million by 2020, a five-year compound annual growth rate (CAGR) of 6.65% through 2020.
Related Conferences:
9th Pharmacovigilance Exhibition, June 21-22, 2018 at London, UK; 12th Pharmaceutical Sciences and Pharma Industry Conference February 26- 27, 2018, London, UK; 7th European Biosimilars Conference, April 26-27, 2018, Rome, Italy; 10th Pharmacology Conferences during Jul 30-Aug 01, 2018 at Barcelona, Spain; 4th Marine Drugs and Natural Products Conferences, June 11-13, 2018 at Rome, Italy; 8th Environmental Chemistry Conference, September 20-22, 2018 Berlin, Germany; 4th Antibiotics Conference; June 14-15, 2018, Barcelona, Spain
4th Annual Pharmacovigilance, Regulatory Affairs, Risk Management and Clinical Trials Conference during May 22-23, 2018 at London, UK; The European Drug Safety Summit during March 7-8, 2018 at London, UK; 19th Drug Discovery Summit during June 2018 at Berlin, Germany; European Regulatory Affairs Forum during May 16-17, 2018 at Brussels, Belgium.
Related societies:
Pharmaceutical & Bioscience Society(PBS), Royal Pharmaceutical Society(RPS), Bangladesh Pharmaceutical Society(BPS), Society for Pharmaceutical Dissolution Science(SPDC), Vancouver Pharmaceutical & Bioscience Society(VPBS), Chinese American Biopharmaceutical Society(CABPS), Pharmaceutical Society of Ghana(PSG), Academy Of Pharmaceutical Sciences(APS)
Track 14 .Opportunities in Pharmaceutical Management
This session of “Pharmatech” focusses on Pharmaceutical Management which is learning about the management and business angle of the health care and the pharmaceutical industry. It gives a strong focus on the basic sciences, combining biological and pharmaceutical sciences with general management studies and marketing.
Pharmaceutical management professionals make financial and business decisions regarding the research and development of noval medicine, marketing, and sales. They take decisions about which types of medication should be developed and how they will be marketed to the public once they have been approved by the Food and Drug Administration(FDA).
Pharma marketing worth $ 28.66 Billion By 2022 | CAGR: 15.8%
The global Pharmaceutical market is expected to reach USD 28.66 Billion by 2022 at a CAGR of 15.8%.
2nd International Conference and Exhibition on Pharmaceutical Development and Technology
May 11-12, 2018 Osaka, Japan
Scope and Importance:-
Pharmaceutical Technology is a latest technological platform to aid efficient drug discovery and development. These platforms range from vast chemical libraries, ultra-high throughput screening and huge genetic databases in discovery, to predictive toxicology platforms and even deep-seated knowledge of particular therapeutic areas in development. New research platforms designed to help its teams of investigators focus on a set of key strategic initiatives that will help guide their multibillion-dollar drug development program to advance new medicines that can get ahead of a disease and prevent it from taking a toll .Pharmaceutical research indulges in making medicines from plant- and chemical-based compounds. They work to prevent the spread of disease, ease pain, cure illnesses, and slow the effects of aging, among many other things. Pharmaceutical companies' mission to discover the next ground breaking medicine is a big cause steeped in big bucks, big competition, and, at times, big controversy. Insiders refer to the handful of multinational giants that dominate the industry-Pfizer and GlaxoSmithKline, to name two of the largest-as Big Pharma.
Branches of Pharmacy Includes:
- Pharmaceutical Sciences
- Pharmaceutics
- Pharmacy Practice
- Pharmaceutical Technology
- Pharmaceutical Development
- Pharmaceutical Marketing
- Pharmaceutical Management
- Drug Discovery
- Drug Design
- Drug Development
About Venue:
Japan is the second-largest individual pharmaceutical market in the world. It accounts for less than 10% of the total global pharmaceutical market. The country’s pharma market is expected to grow annually by ~2.2%. Shionogi & Co's new pharmaceutical research facility is located in Toyonaka City in Osaka, Japan. Osaka is a large port city and commercial centre on the Japanese island of Honshu. Osaka is famous for modern architecture, nightlife and hearty street food. The 16th-century shogunate Osaka Castle, which has undergone several restorations, is its main historical landmark. It's surrounded by a moat and park with plum, peach and cherry-blossom trees. Sumiyoshi-taisha is among Japan’s oldest Shinto shrines.
Why to Attend???
- With members from around the world focused on learning about innovations, latest technologies, different drug formulations for effective treatment and new drug moieties through pharmaceutical technology and its importance, this is your best opportunity to reach the largest assemblage of participants from the Clinical Research organizations, Pharmaceutical companies and technology related societies Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new research developments, and receive name recognition at this 3-day event. World-renowned speakers, the most recent issues, approaches, and the novel trends in Pharmaceutical Technology are hallmarks of this conference
- Major sessions included at this conference are Pre-formulation studies, Drug Formulation Procedures, Drug Product Manufacturing, Pharmaceutical Engineering, Pharmaceutical Manufacturing, Pharmaceutical Technology, Technologies in Drug Delivery, Types of Pharmaceutical Formulations, Regulatory Requirements for Pharmaceuticals, Thermodynamics, Pharmaceutical Management.
Target Audience:
- Pharmaceutical, Biomedical and Biotechnology industries
- Associations and societies related to Pharma & Biomedical field
- Students and Professors from the academia of the various universities
- Researchers, Scientists, Experts, Business delegates in the field of Pharmaceutical & Biomedical Sciences.
Societies/Industries/Universities Associated with Pharmaceutical Technology and Development:-
- International Society of Pharmaceutical Compounding (ISPhC)
- International Young Pharmacists' Group (YPG)
- Parenteral Drug Association (PDA)
- Regulatory Affairs Professionals Society (RAPS)
- Society for Biomolecular Sciences (SBA)
- Society for Cell Science (SFCS)
- Al-Hayat Pharmaceuticals
- Amgen
- Elis Pharmaceuticals Limited
- Alliance Global
- Sanofi Aventis UAE
- Genpharm
- Ranbaxy Laboratories Limited
- BioPharma
- Hokkaido University
- Waseda University
- Nagoya University
Major Pharmaceutical Tchnology Societies Around The Globe
- Controlled Release Society (CRS)
- Institute of Pharmacy Management International (IPMI)
- International Academy of Compounding Pharmacists (IACP)
- International Association for Pharmaceutical Technology (APV)
- International eHealth Association
- International Federation of Pharmaceutical Manufacturers Associations (IFPMA)
- International Federation of Pharmaceutical Wholesalers (IFPW)
- International Pharmaceutical Excipients Council (IPEC)
- International Pharmaceutical Federation (FIP)
- International Pharmaceutical Students Federation (IPSF)
- International Society for Medical Publication Professionals (ISMPP)
- International Society of Oncology Pharmacy Practitioners (ISOPP)
- International Society for Pharmaceutical Engineering (ISPE)
- International Society for Pharmacoepidemiology (ISPE)
- International Society for the History of Pharmacy (ISHP)
- International Society for the Study of Xenobiotics (ISS
Major Pharmaceutical Technology Societies in Asia
- Federation of Asian Pharmaceutical Associations (FAPA)
- Asia Partnership Conference of Pharmaceutical Associations(APAC)
Target Audience:
Industry 50.3%
Academia 38.7%
Others 11%
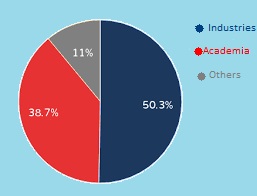
Figure 1: Target Audience
Top Universities in Japan:
- Tohoku University
- University of Tokyo
- Kyoto University
- Osaka University
- Tokyo Institute of Technology
- Kyushu University
- Hokkaido University
- Nagoya University
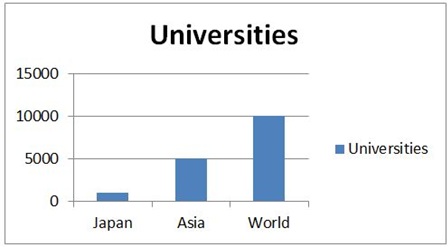
Figure 2: Statistical Analysis of Universities
Market Value on Pharmaceutical Research:-
The global pharmaceutical contract manufacturing/contract research market is anticipated to reach USD 238.3 billionby 2025, according to a new report by Grand View Research, Inc. Impact on the volume of capacity that is required for production due to of increasing expression levels is anticipated to influence outsourcing industry. Increased cell densities as well as increase in expression of each cell has led to the steady decline in the volume requirement. The aforementioned fact provides the client with choices for in selection from several CMOs, which was not the fact earlier.
The focus of large pharmaceutical companies to strategically engage with a small number of preferred providers is a meaningful departure from the procurement-based outsourcing. This was one of the many approaches that the service industry had become accustomed to.
Expanding market for biosimilars and biobetters is anticipated to pronounce the demand for contract development and manufacturing support. Service providers that hold the potential of providing cost as well as quality advantages are set to make inroads and grow at tremendous pace in the forecast period.
However, as drug manufacturers are increasing the investments with respect to production capabilities. This may limit the potential growth of contract manufacturing services to traditional customers thereby hampering industrial growth.
Market Growth of Pharmaceutical Research:
Overall sales in the Pharmaceutical market amounted to $34.2 billion in 2007, and decreased slightly to $31.1 billion in 2009. By 2014, they are projected to reach $32.2 billion, for a 5-year compound annual growth rate (CAGR) of 0.7%.
The largest segment of the market, prescription drugs, was valued at $27.4 billion in 2008; this is expected to decrease to $26,3 billion in 2009, and to reach $27 billion in 2014, for a 5-year CAGR of 0.5%.The global excipients market was at $4.6 billion in 2010 and it is expected to reach at $4.9 billion in 2011. It is further anticipated to increase to $6.7 billion by 2016 at a compound annual growth rate (CAGR) of 6.5%.
Funds allotted to Pharmaceutical Research:-
Worldwide:
Pharmaceutical organizations have generally financed their inward R&D endeavours absolutely all alone by contributing 15% or a greater amount of their top line incomes to pay for these attempts. Likewise, keeping in mind the end goal to take advantage of R&D going ahead outside their organizations, pharmaceutical organizations has monetarily upheld outer R&D endeavours in manages little biotech organizations, inquire about foundations, and colleges. Indeed, as subsidizing for organizations like the NIH has stagnated over late years, Pharma has turned into a critical wellspring of assets to bolster early stage look into.
The R&D Funding Forecast takes note of that there is a proceeding with move in where R&D speculations are being made, with less in the U.S. also, Europe and more in Asian nations. The U.S. presently represents not exactly 33% of worldwide spending, while Europe's 34 nations represent under 22% and Asian nations represent about 40%, a pattern that has proceeded for as long as five years. This pattern is relied upon to proceed through the end of the decade with China's R&D ventures outperforming those of the U.S. by around 2022.
Asia:
Science and innovation is booming in Asia, and going about as a magnet for Asian researchers wishing to return home in the wake of preparing in the West particularly to China-pulled in to full or low maintenance positions in both scholarly world and industry.
Advancing the eastbound movement is a solid government push-especially in China, Singapore, Korea, Taiwan and Japan-to end up worldwide players in science and innovation, and enormous speculation from the pharmaceutical business. The outcome is an overwhelming blend of new R&D openings.
Apart from the industrial personnel where most of the research work is done, other research communities include:-
- Academicians include Student community.
- Researchers include Post docs, Research Associates.
- Scientists include Professors, Associate professors, and Assistant professor.
- Industries include Presidents, CEO’s, and R&D Managers.
Market Report of Pharmaceuticals:
- The worldwide market for pharmaceuticals is projected to grow from around $1 trillion in 2015 to $1.3 trillion by 2020, representing an annual growth rate of 4.9 percent.
- Several global demographic and economic trends are driving pharmaceutical consumption, including a rapidly aging world population and an associated rise in chronic diseases, increased urbanization and higher disposable incomes, greater government expenditure on healthcare and growing demand for more effective treatments.
Pharmaceutical technology:
- The Indian pharmaceuticals market increased at a CAGR of 17.46 per cent during 2005-16 with the market increasing from US$ 6 billion in 2005 to US$ 36.7 billion in 2016 and is expected to expand at a CAGR of 15.92 per cent to US$ 55 billion by 2020.
- By 2020, India is likely to be among the top three pharmaceutical markets by incremental growth and sixth largest market globally in absolute size.
- India’s cost of production is significantly lower than that of the US and almost half of that of Europe. It gives a competitive edge to India over others.
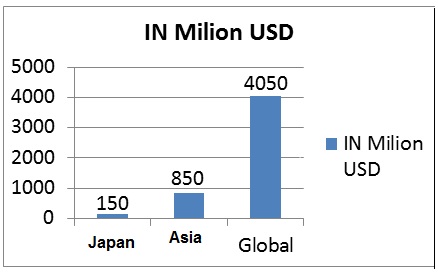
Figure 3: Growth forecast for Pharmaceutical Technology
Pharmaceutical Materials :
Growing urbanization across the globe, increasing affluence in the Asia-Pacific region, and rising demand for improved and efficient healthcare services, globally are the major drivers for the growth of the pharmaceutical packaging market. In addition to this, stringent government regulations related to manufacturing of counterfeit medicines in several countries of the world are also expected to fuel the growth of the pharmaceutical packaging market during the forecast period, 2014 to 2020.
Figure 4: Growth forecast of materials
Drug Discovery and Design:
- Analyses of global market trends, with data from 2012, estimates for 2013, and projections of compound annual growth rates (CAGRs) through 2018.
- Examinations of major issues involved in the research and development (R&D) of more effective cell-based approaches, for drug discovery, and absorption, distribution, metabolism, and excretion (ADME)/ toxicity assays in development and in use.
- Discussion of current issues and trends affecting the industry, and costs and other factors influencing demand.
- Coverage of new technologies, trends, alliances, and mergers.
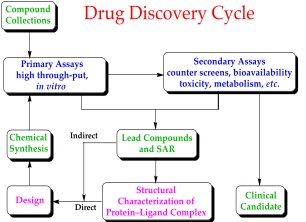
Figure 5: Drug Discovery Cycle
Drug delivery:
- The global drug delivery technology market is projected to reach USD 1,669.40 Billion by 2021 from USD 1,179.20 Billion in 2016, at a CAGR of 7.2% during the forecast period. This market is segmented based on route of administration, facility of use, and region.
- Rising prevalence of chronic diseases, growth in biologics market, technological advancements and new product launches are some of the major factors driving the growth of the market. Drug delivery technologies improve the efficacy and safety of a drug by controlling the rate, time, and place of drug release in the body.
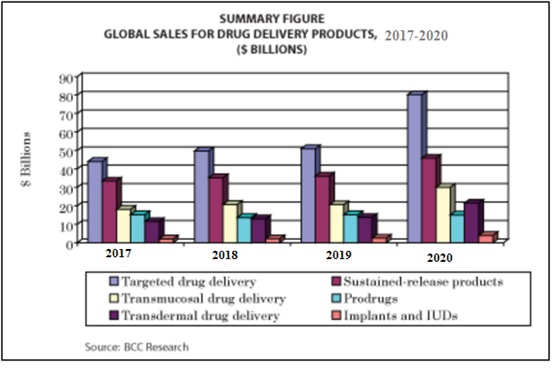
Figure 6: Drug Delivery Global sales
Pharmaceutical BioTechnology:
BioPharma is one of the world's most profitable industries. During the last 30 years, the industry has spent billions of dollars on research and reaped billions in return. In 2006 alone, the pharmaceutical industry introduced 31 major drugs and sold $643 billion in products worldwide-a 7 percent increase over 2005 sales, according to the drug market research firm IMS Health. U.S. sales beat the national average with growth of 8.3 percent.
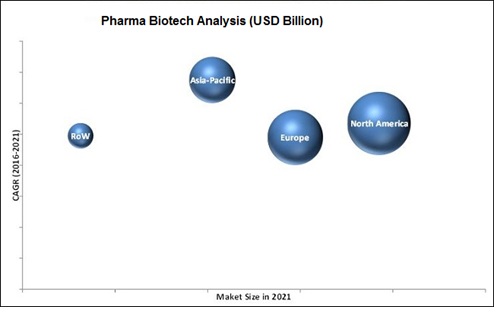
Figure 7: BioTech Analysis
Pharmaceutical Nanotechnology:
- According to BCC Research (www.bccresearch.com), the global market for nanoparticles in the life sciences is estimated at over $29.6 billion for 2014. This market is forecast to grow to more than $79.8 billion by 2019, to register a healthy compound annual growth rate (CAGR) of 22%. The biggest increase will come in the area of drug delivery systems.
- As products complete clinical trials and gain US FDA market approval, the revenues from these products will grow at 23%. Basic biotechnology research revenues will increase due to the quest to find more nanoparticle applications, as more drugs become successfully delivered by these carrier systems.
- Drug development and formulation will show steady sustained growth at 20.7%. Nanoparticles for use in diagnostic imaging will continue to show healthy growth at 20.1%. This will result from the need to develop more definitive nanoparticle markets for disease diagnosis.
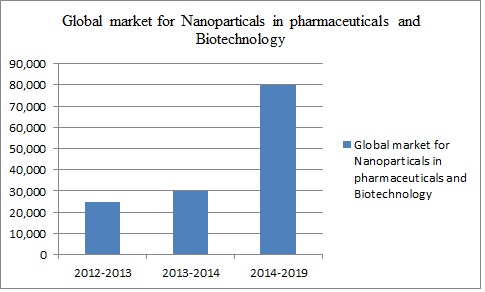
Figure 8: Global Market for Nanoparticles in Pharmaceuticals
Drug Regulatory Affairs:
- More than 15 years span is required to develop and launch a new pharmaceutical product in the market. The Regulatory Affairs Agencies explains expenditure spent on each drug annually is $850 million in US
-
Pharmaceutical industry is one of the fastest growing industries in India, with a compounded annual growth rate (CAGR) of over 13 % in last 5 years and it is expected to grow at a higher rate in coming 10 years where India spends around $300 million, united kingdom $700 , Brazil $200 million , France $550, Italy $400. As per cabinet decision in June 2010 Japan amended a new policy i.e., Health power strategy through “Life Innovation” with an expenditure spent on each drug was $450 million.
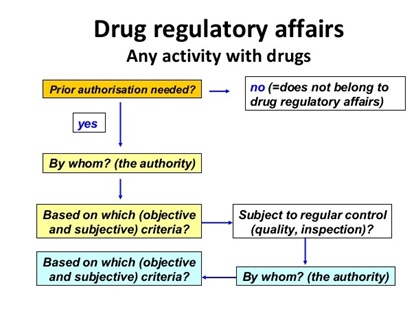
Figure 9: Drug regulatory affairs
Medical Devices:
- The global medical devices (MD) market will see a steady growth over the coming years with the sales revenue and international trade value reaching $543.9 billion and $289.2 billion respectively by 2020 driven by aging population, increasing healthcare expenditure and technology advancement.
- Global Medical Devices Market 2012-2020 examines the worldwide market of medical devices through a comprehensive review of information sources. This report provides historical performance, in-depth analysis, and trend forecast of market size and share, sales revenue, international trade, R&D investment, geographic distributions and product categories by device area in the global medical device market and industry. Historical statistics cover the past three years and forecast data characterize the 2014-2020 period.
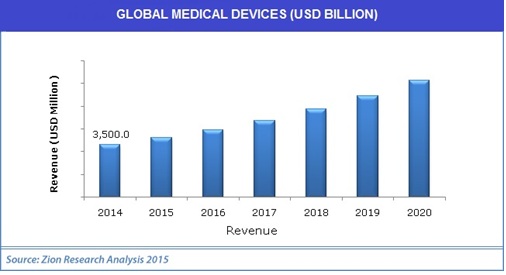
Figure 10: Global Market for Medical Devices
*Source: BCC Research & Markets and Market
Conference Highlights
- Recent developments In Pharmaceutics & Biopharmaceutics
- Advancements In Drug Targeting & Drug Development
- Scope Of Pharmaceutical Research
- Recent Development Of Pharmaceutical Materials
- Challenges In Pharmaceutical Formulations
- Advancements In Pharmaceutical Biotechnology
- Approaches In Pharmaceutical Nanotechnology
- Innovations In Pharmaceutical Technology
- Advancements In Pharmaceutical Manufacturing
- Development Of Pharmaceutical Engineering
- Evolving Of Medical Devices
- Advancement Of Drug Regulatory Affairs
- Opportunities In Pharmaceutical Management
- Innovation Of Drug Delivery Technologies
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | May 11-12, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
Abstracts will be provided with Digital Object Identifier by














































